BIOSENSORS, MOTOR PROTEINS AND MORE
MARTIN WEBB
SCIENCE
Home > Research topics > Myosin
MYOSINS AND MUSCLE
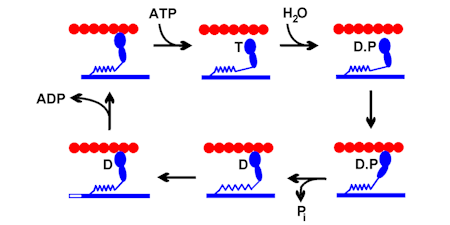
INTRODUCTION
There has been interest in the laboratory over many years in energy transduction by myosins, originally that of skeletal muscle but more recently with non-conventional myosins.
Work on this system has centred on the chemical and kinetic mechanism of ATP hydrolysis, and on how this is coupled to movement and force. This mechanochemical coupling was investigated in myofibrils and muscle fibres, in which there are organised filaments to produce tension and contraction.
The first work on myosin was aimed at understanding the chemistry of ATP hydrolysis. There was a general problem of relating hydrolysis mechanisms in solution to what happens in any ATPase catalytic site and defining biochemical states as particular chemical species. For example, the “cleavage step” was defined on the basis that the product of that step gave ADP + inorganic phosphate (Pi) on quenching and kinetic measurements suggested that this step was a single process.
But several mechanisms could not be distinguished, such as reaction via a short lived phosphoenzyme, a dissociative mechanism (producing metaphosphate - PO3- – as first product in the myosin site) or an associative one (producing pentacovalent intermediate).
Resolving this was important for relating defined biochemical intermediates with structural states and understanding how the energetics of the ATPase pathway coupled to force generation. We now know that the reaction is in-line displacement of ADP by water oxygen (“Sn2”) and that information could be fed into our understanding of crystal structures as they emerged.(Ref 18, 43)
I would like to acknowledge and thank all the people from my lab and elsewhere, who contributed to this work. Their names are on the cited publications.
ATPASE MECHANISM 1
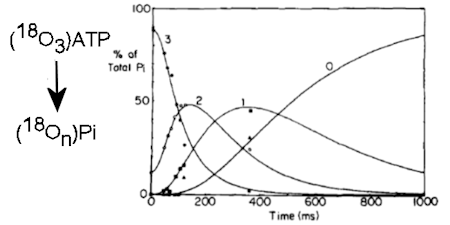
In order to probe the chemistry, particular use was made of adenosine nucleotides, substituted at particular positions with stable oxygen isotopes and part of the work required novel strategies to get site-specific substitution. Such labeling was useful to probe cleavage reversibility, synchronisation of ADP-gammaP bond cleavage with H2O-gammaP bond formation, the stereochemistry of this substitution, Pi release kinetics, and metal ion coordination.
Phosphate-water oxygen exchange had been established previously as a powerful way to probe ATPase reactions, particularly Pi release and cleavage reversibility. Incubation of (18O4)Pi with myosin S1 and ADP and following the exchange in real time allowed gave a rate for Pi binding and set a limit on the rate constant for the ATP resynthesis. This method was used to measure the rates of binding of Pi anto the ADP complex and then on-enzyme synthesis of ATP, thereby showing complete reversibility of the part of the ATPase reaction. (Ref 5, 37)
The methodology was then extended by combining with the rapid reaction technique of quenched-flow, so the fate of individual oxygens could be followed during a single turnover of ATP on myosin. This showed that it was very likely that a water oxygen was fully incorporated in the ADP.Pi complex and that this oxygen, together with the three from the gamma-P of ATP are fully randomized in the bound ADP.Pi. (Ref 12)
With an alternative isotope exchange protocol (PIX) using oxygen-18 in the beta-phosphate, it was shown that ADP is fully released from the gammaP. Together, these results provided strong evidence for the chemical nature of key intermediates during myosin-catalysed ATP hydrolysis and for ADP and Pi being present as separate species in this products state. (Ref 7,9)
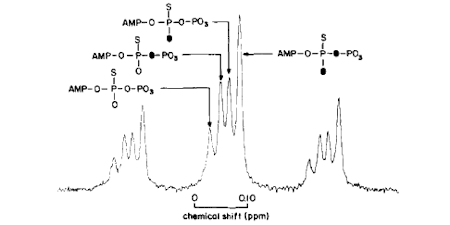
ATPASE MECHANISM 2
A general issue of the chemical mechanism of ATPases is whether there is a phosphoenzyme intermediate as opposed to direct transfer of phosphate. Such a phosphoenzyme may be present only transiently and so not in sufficient amount to identify directly. The stereochemical course of phosphotransfer reactions was shown to provide a method that could distinguish the number of phosphotransfer reactions in an enzyme-catalysed pathway. This showed that myosin ATPase proceeds via an in-line displacement of ADP by water: no phosphoenzyme. (Ref 6, 8, 15)
Understanding the chemistry of ATP hydrolysis also requires consideration of the metal coordination during the reaction. Prior to crystal structures of each intermediate, signals giving information about metal coordination were required. Because of the established expertise with oxygen isotopes, this was investigated using 17O-hyperfine splitting of Mn EPR. The method defined the coordination of water and phosphate oxygens with ADP and ADP.Pi complexes and showed that the presumed beta-gamma-coordination in the ATP state was maintained in the ADP.Pi state. This neutralization of charge on the ADP helps facilitate bond cleavage, while coordination with the gamma-P then Pi contributes to the catalytic attack by water (Ref 17).
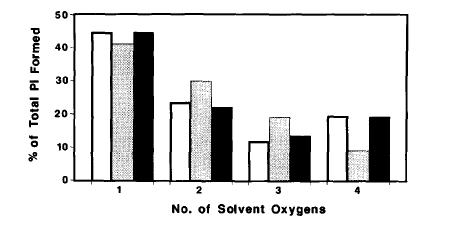
MECHANOCHEMICAL COUPLING 1
Later work in the field of myosin motors has concentrated on understanding how the ATP hydrolysis cycle was altered at different levels of organization of proteins from actomyosin in solution to muscle fibres and then how the ATPase cycle is coupled to force or movement generation. These were in collaboration with physiologists, as in the citations.
Oxygen exchange techniques were used to study changes in individual steps of the cycle at higher levels of organization, particularly in fibres: this was one of the few techniques that could be used in such preparations to measure kinetics until the establishment of the phosphate biosensor. This work provided direct evidence that Pi can bind in the catalytic site of myosin in fibres and so cause the known effects on tension. Oxygen exchange revealed that the ATP cleavage step is readily reversible, as in isolated proteins, and is followed by Pi release, which is promoted by fibre activation. This provided a kinetic test that fibre activation by calcium leads to greater access to actin by myosin.(Ref 21, 23, 27, 35)
However, the rabbit skeletal fibre data indicated multiple kinetic pathways, potentially due to different myosins having different degrees of access to actin. Use of insect flight fibres provided a system to examine this through use of strain activation of this muscle type. Oxygen exchange again showed activation of Pi release by activation, but now showed essentially a single pathway of ATP hydrolysis, suggesting all myosins can interact similarly with actin. This also confirmed Pi release as rate limiting in the ATPase pathway. (Ref 22, 28, 30, 40)
The oxygen exchange technique was extended to give information about reversibility of ATP binding in both actomyosin in solution and in fibres. These data allowed development of a model, in which the fibre organization produced a distribution of strains in individual cross-bridges, modifying the kinetics from those observed in solution in a defined way by the degree of distortion by strain. (Ref 35, 37)
MECHANOCHEMICAL COUPLING 2
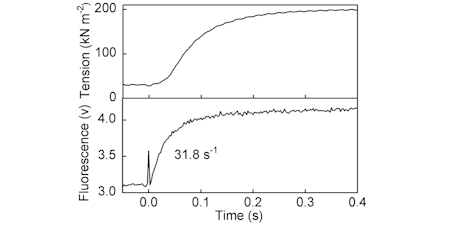
These measurements on fibres provided the impetus the original development of the phosphate biosensor to provide a novel and direct way to investigate the otherwise intractable phosphate release kinetics, a key process in the cycle relating to the force-generating power stroke.
Transient measurement of Pi release was shown to be concomitant with formation of most of the tension, during fibre activation. This led to a study on ATP usage and therefore showing efficiency of 0.36 (relative to free energy available) during activation of force. (Ref 50, 56, 68, 83)
This method could also be applied to myofibrils, a preparation of organised muscle proteins for which both shortening measurements and rapid-mixing experiments could be performed. (Ref 52, 58, 79)
Until the developments of the phosphate biosensor, there was not a method to measure Pi release from motor proteins in real time. The Pi release kinetics of actomyosin were measured directly using double-mixing stopped-flow and shown to be similar to that in contracting fibres. (Ref 57)
FLUORESCENT NUCLEOTIDES AND NON-MUSCLE MYOSINS
A coumarin-ATP adduct has being used as part of a collaboration for fluorescence lifetime imaging to detect actomyosin states in fibres.. Lifetimes not only vary with nucleotide state, but also with the external force on a fibre, showing the interplay of small changes near the ATP site and gross changes to the myofibril. (Ref 91, 107)
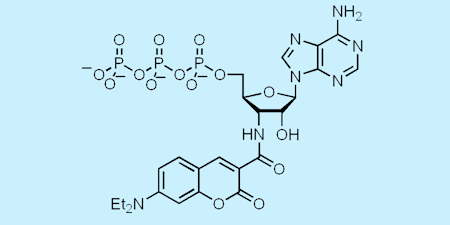
More recently, another of our fluorescent analogues of ATP, diethylaminocoumarin-aminoATP gave a large increase in fluorescence (up to twenty-fold) on binding to various myosins. That enabled study of diphosphate release in myosin V in a collaborative project: myosin V is a highly processive motor, taking cargoes along actin filaments in cells by a stepping mechanism. (Ref 90, 93, 96)
Using this analogue, it was shown that the dissociation from the trail head is 250-fold slower than that from the leading head, thereby providing a direct role for nucleotide processing in holding the leading head in place while the trailing head steps past it. This led to a single-molecule study, in which observation of coumarin fluorescence (both movement and intensity) using this nucleotide analogue. This provided direct evidence of the stepping mechanism and its relation to the state of bound nucleotides. (Ref 93)